Focused expertise in all facets of drug development
From discovery and planning all the way through regulatory, we support small biotech’s with complete and comprehensive drug development — delivering the strategy, team and roadmap clients need to be successful.
- We create efficiencies by knowing just how to navigate each phase of drug development
- We help companies with limited budgets maximize resources and create value
- We rely on a team – 150 associates who satisfy every niche of virtual drug development
- We provide expert advice steeped in real-world drug development experience
- We work hands-on to achieve desired biological outcomes
- We can provide a full team or simply the individual expert needed which makes us ready from day one
Designing and implementing a comprehensive drug development program.
Yes, we can do that.
Drug Discovery
- Medicinal Chemistry
- Lead Optimization
- Target Product Profile
Strategic Planning
- Defining the Program
- Outlining non-clinical, clinical and regulatory strategies
- Developing a timeline and budget
Non-Clinical Development
- Disposition, Metabolism, and Pharmacokinetics (DMPK)
- Non-clinical pharmacology
- Safety pharmacology
- Toxicology
Drug Substance Development
- Identifying Vendors
- Developing scalable synthesis route
- Analytical method development
- GMP manufacturing
Drug Product Development
- Dosage form selection
- Formulation development
- Analytical development
- GMP manufacturing
Regulatory
- Regulatory strategy
- Pre-IND meeting and briefing book
- IND (and/or IMPD) preparation and filing
Management
- Program leadership
- Project management
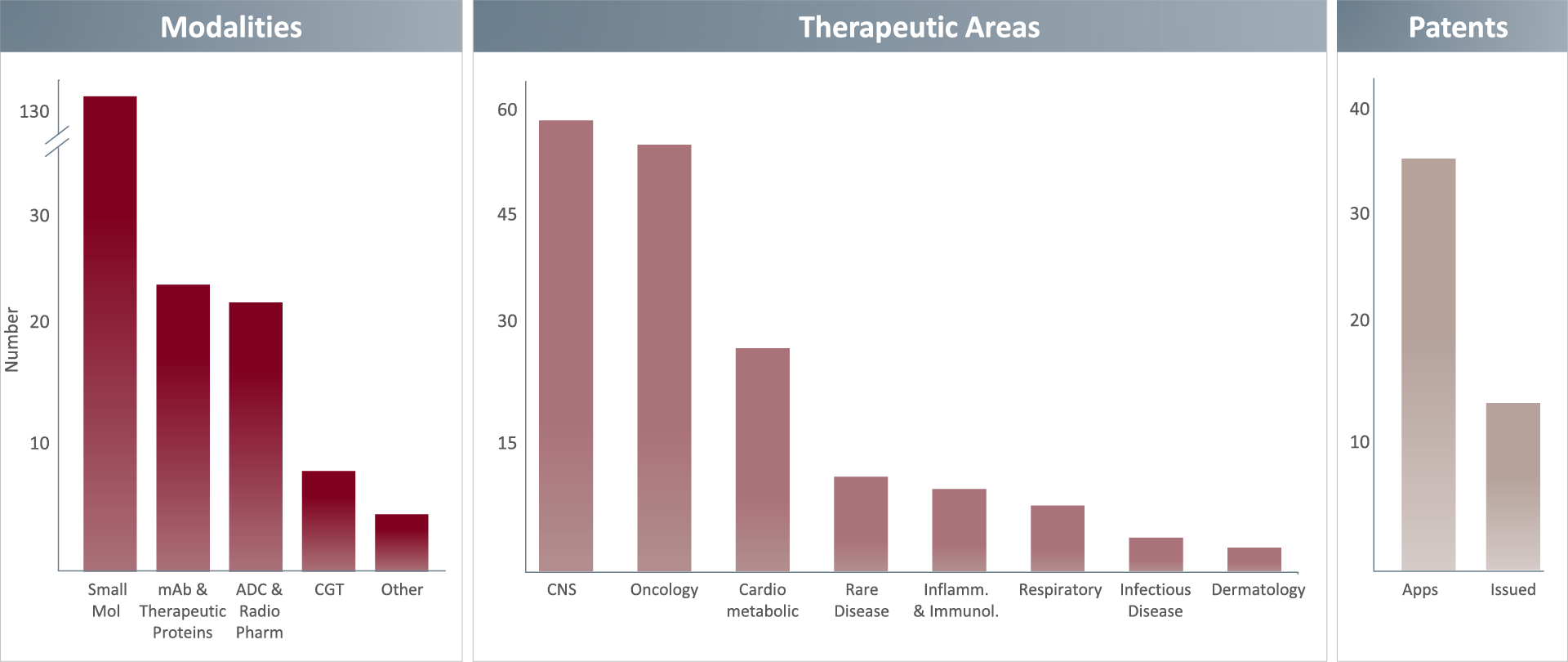
We’ve worked across diverse therapeutic areas & across multiple modalities
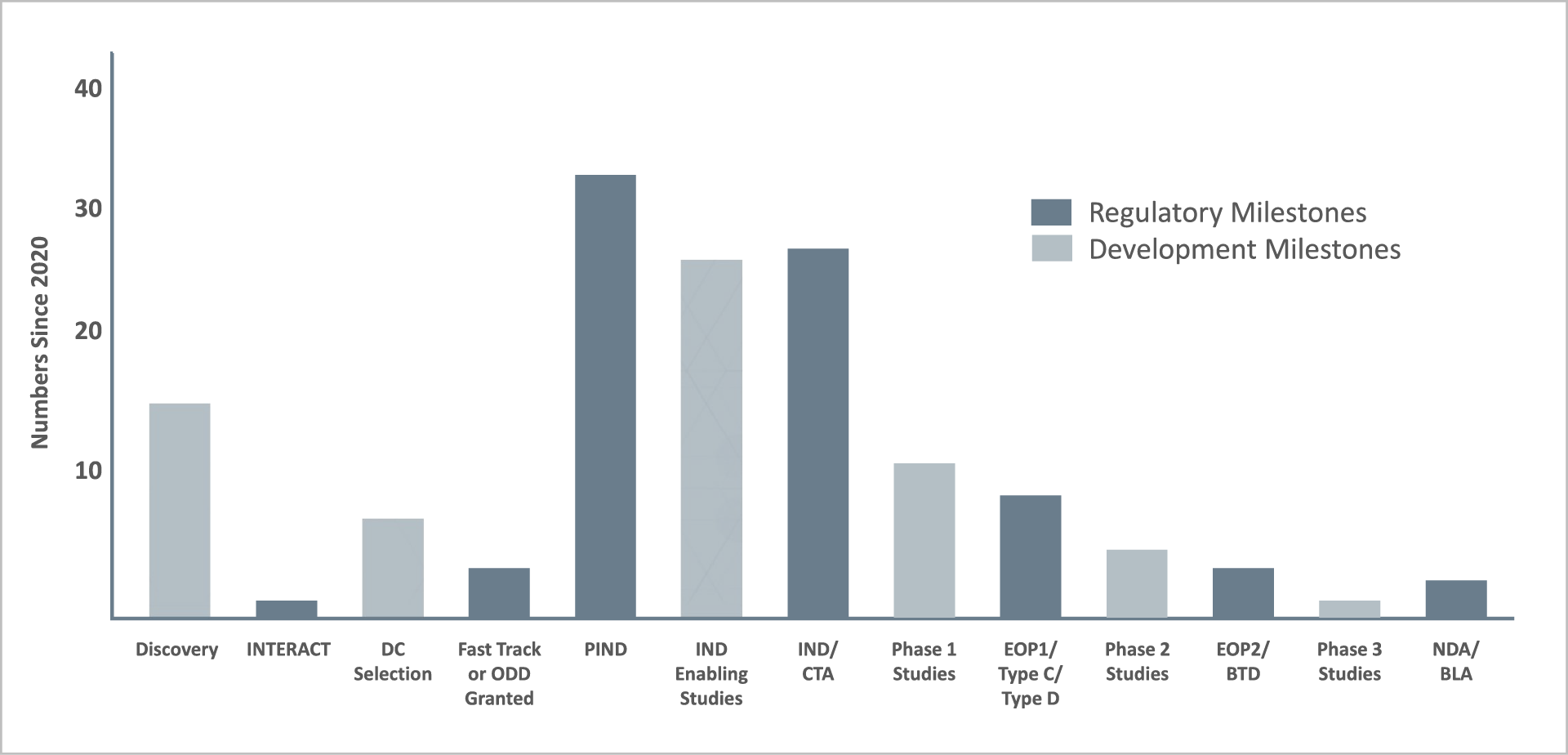
We work across the full spectrum of drug development and regulatory milestones
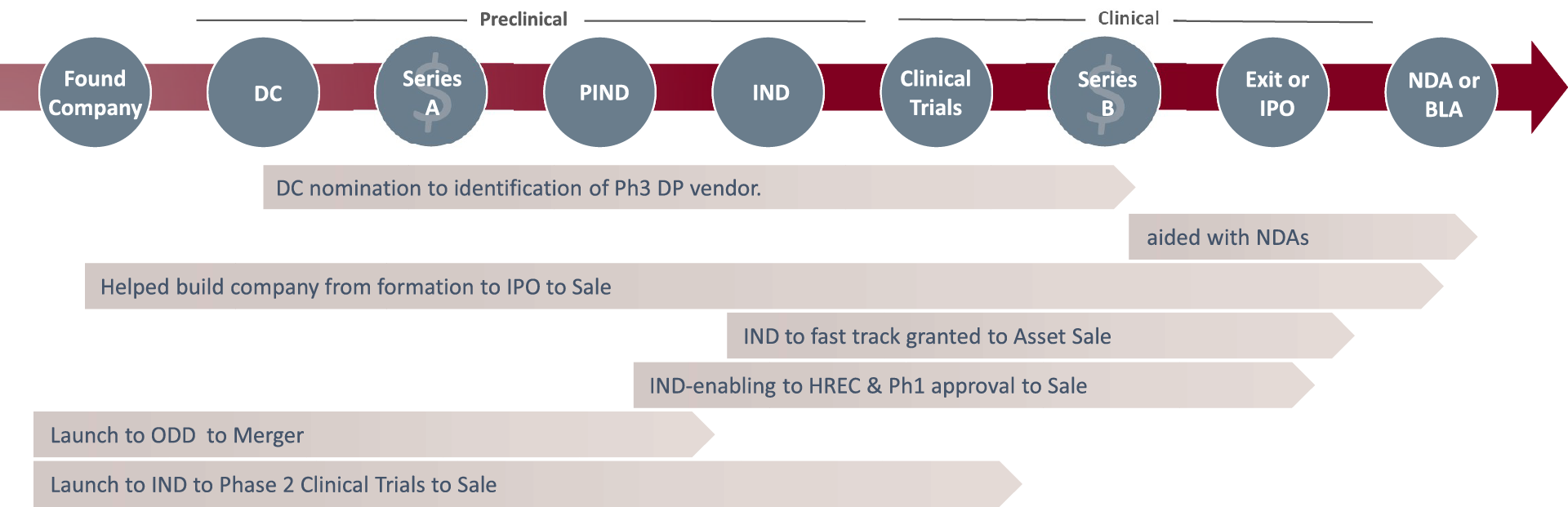
Company Lifecycle
We integrate at diverse stages and enable cost efficient progression to the next stages.
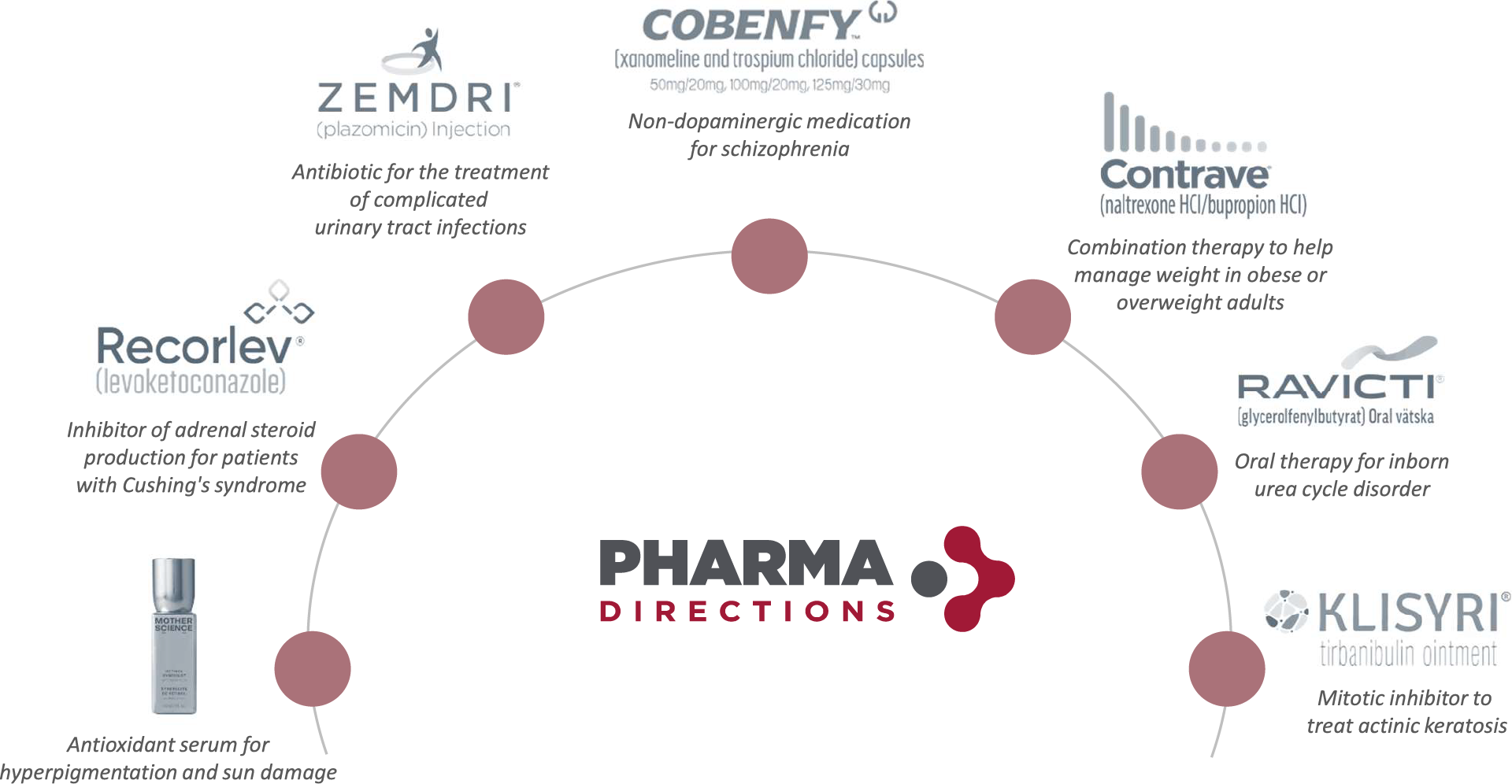
Marketed products we’ve helped develop